SPC Blog
What If Subgroup Sizes Are Not Fixed?
In standard Statistical Process Control (SPC) textbooks, subgroup sizes are typically recommended to be fixed at 3–5 samples.
This recommendation is based on an ideal assumption: each production batch or time interval yields the same number of samples.
However, in real manufacturing environments, this assumption often does not hold.
Due to factors such as end-of-batch material shortages, sample loss, or varying time windows in high-frequency automated data collection, subgroup sizes (n) frequently fluctuate.
Limitations of Traditional SPC Tools
When faced with variable subgroup sizes, traditional Excel templates or entry-level SPC software usually fail—either producing errors or requiring manual data splitting and padding.
Such workarounds not only distort the authenticity of the data, but also obscure the true sources and structure of process variation.
How Simple SPC Handles Variable Subgroup Sizes
Simple SPC provides full support for SPC analysis with non-fixed subgroup sizes using statistically sound and production-ready methods.
Stepwise Control Limit Algorithm
Accurately Capturing the True Voice of the Process
From a statistical standpoint, variations in subgroup size (n) directly affect the standard deviation of the subgroup mean.
For this reason, Simple SPC dynamically calculates the Upper and Lower Control Limits (UCL/LCL for each individual data point, based on the actual subgroup size of that point.
As a result, the control chart displays scientifically derived stepwise control limits, ensuring that out-of-control detection for every subgroup is statistically rigorous, consistent, and reliable, even when subgroup sizes fluctuate.
Full Control Chart Support for Variable Subgroups
For processes with fluctuating subgroup sizes, the system provides a complete set of statistical tools:
X-bar Chart
Monitors the process mean and central tendency.R Chart / S Chart
Monitors within-subgroup variation.
When subgroup sizes vary significantly, the system recommends using the Xbar-S chart, as it utilizes all sample information more accurately to estimate process variation.
Process Capability Analysis
CPK / PPK
- PPM
Distribution plots
Capability histograms
Example
The figure below shows an SPC analysis report for a process with variable subgroup sizes generated by Our SPC.
Using the same dataset, the results were recalculated using the Simple SPC CPK Tool for verification and comparison.
CPK Tool Support
The CPK Tool fully supports analysis based on variable subgroup sizes, ensuring consistency between SPC monitoring and capability evaluation.
Consistent with Minitab Results
Professional rigor is the bottom line for quality engineers.
To validate statistical accuracy, we input the same complex dataset with variable subgroup sizes into both Minitab and the Simple SPC 4.0 CPK Tool for parallel verification.
Validation Results
The results show that both systems produce completely identical outputs, including:
Control limits (UCL / LCL)
Mean values
Sigma estimates
Process capability indices (CPK, PPK)
This confirms that Simple SPC maintains industrial-grade statistical precision, while delivering a lightweight, fully digitalized experience through a browser-based (B/S) architecture with no client installation required.
Invisible Variations That Define Yield :Practical Applications of SPC in Semiconductor Manufacturing
·Process Control Methods for High Yield and Stable Volume Production:
1. What Is the Real Risk in Semiconductor Manufacturing?
In semiconductor manufacturing, the most dangerous issues are often not obvious out-of-spec events, but invisible and persistent process variations.
Wafer fabrication involves hundreds to thousands of tightly coupled process steps, each operating within extremely narrow process windows. Even a minor parameter shift can be amplified through downstream processes, eventually resulting in:
Yield degradation
Parameter distribution drift
Large-scale scrapping of high-value wafer lots
This reality determines that the semiconductor industry cannot rely on end-of-line inspection to ensure quality. Instead, it must continuously answer two critical questions during production:
Is the process stable?
Is the process still under control?
Statistical Process Control (SPC) exists precisely to address these questions and has become a foundational capability in modern semiconductor manufacturing.
2. The Role of SPC in the Semiconductor Industry
Unlike traditional manufacturing, SPC in semiconductor fabs is not merely a statistical tool used by quality departments. Instead, it serves as:
A daily monitoring method for process engineers
A key reference for equipment and process condition assessment
A critical input for yield management and production release decisions
In practice, SPC is typically integrated with MES/EAP/FDC/APC systems, forming a comprehensive process control framework that supports:
Early identification of process drift
Proactive exception handling in advance
Support process and equipment decisions
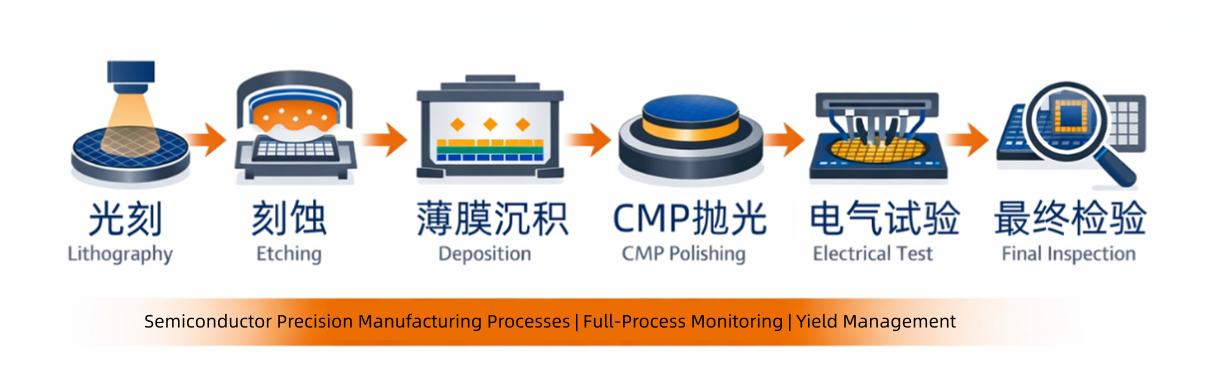
3. Typical SPC Application Scenarios in Semiconductor Manufacturing
3.1 Lithography: Core Control of Process Windows
Lithography is one of the most yield-critical steps in semiconductor manufacturing. SPC is commonly used to monitor:
Critical Dimension (CD)
Overlay
Dose
Focus
Given the extreme sensitivity of lithography parameters to yield, semiconductor fabs focus heavily on subtle trend shifts rather than obvious limit violations. Therefore, SPC applications often combine:
I-MR control charts
EWMA and CUSUM trend detection methods
to enable early detection of ”chronic loss of control“.
3.2 Etching: Ensuring Uniformity and Stability
In etching processes, SPC is primarily applied to monitor:
Etch depth
Line width variation
Within-wafer and wafer-to-wafer uniformity
Continuous SPC monitoring helps engineers identify:
Chamber condition changes
Consumable aging and contamination risks
Process parameter drift
thereby reducing the risk of batch-level excursions.
3.3 Thin Film Deposition (CVD / PVD / ALD): Consistency Management
Typical SPC monitoring parameters in deposition processes include:
Film thickness
Refractive index
Resistivity
Uniformity
SPC is used not only for single-tool stability control, but also widely applied in:
Tool matching across multiple equipment sets
Maintenance and cleaning interval optimization
3.4 CMP (Chemical Mechanical Planarization): Preventing Over-Polish and Under-Polish
CMP processes are characterized by high process noise and complex parameter coupling. SPC monitoring focuses on:
Removal rate (RR)
Surface roughness
Planarity metrics
By applying SPC, fabs can distinguish random variation from systematic drift, preventing long-term yield loss caused by cumulative deviations.
3.5 Electrical Testing and Yield Monitoring
In front-end manufacturing, SPC is applied not only to process parameters, but also extensively used for:
Monitoring key electrical characteristics
Analyzing yield trend indicators
This allows engineers to trace yield anomalies upstream to specific process steps, enabling faster root-cause identification.
4. Key Characteristics of SPC in Semiconductor Manufacturing
Compared to traditional manufacturing, semiconductor SPC exhibits distinct characteristics:
4.1 High Frequency, Small Samples, Large Data Volumes
Single-point or very small subgroup sampling
High-frequency monitoring
4.2 Complex Data Distributions
Non-normal distributions are common
Skewed and long-tailed characteristics frequently observed
As a result, practical SPC applications often require a combination of:
Data transformation methods
Trend-based control charts
Non-normal analysis strategies
4.3 Focus on Trends Rather Than Single-Point Violations
In semiconductor manufacturing, the most significant risks typically arise from:
Long-term, gradual, and continuous process drift
Therefore, the core value of SPC lies in early trend detection, rather than reacting only after parameters exceed control limits.
5. Core Value Delivered by SPC for Semiconductor Enterprises
Through systematic SPC implementation, semiconductor manufacturers can:
Detect process instability early and protect yield
Reduce the risk of scrapping high-value wafers
Support equipment maintenance and process optimization decisions
Improve consistency and stability across tools and production lines
In advanced process nodes, SPC has become a key reference for process release and stable mass production.

6. Conclusion: SPC as the “Invisible Defense Line” of Semiconductor Manufacturing
In the semiconductor industry:
Invisible variations are often the greatest risk.
SPC is not merely a set of statistical charts, but a comprehensive process control methodology designed to:
Continuously monitor process conditions
Detect abnormal trends at an early stage
Safeguard yield and stable volume production
Those who can identify process drift earlier are better positioned to protect yield—and maintain long-term competitiveness.
Simple SPC 4.0 – Detailed Product Overview
Simple SPC is a privately deployed, browser-based Web SPC system designed for enterprise-level statistical process control.
No client installation is required—users can access the system directly through a standard web browser.
Bingo SPC has been recognized for three consecutive years as a “Recommended SPC Software” by SoftServe Home (软服之家), a leading Chinese enterprise software evaluation platform.
1. Web-Based Architecture
- Server licensing: One-time lifetime license, no annual licensing fees.
- Browser-based access, no local client installation required
- Unlimited user count, unlimited online users, unlimited points (features, detection items), unlimited dashboards.
- Supports SPC control charts:
- I–MR
- Xbar-S
- Xbar-R
- MR–R/S
- NP, C, P, U
- Supports 11 languages, Simple is the most language support globally, Including English, Simplified Chinese, Traditional Chinese, Spanish, Japanese, Korean, Vietnamese, Indonesian, Hindi, Thai, and Malay.
- All company employees can use it.
2. Rule-Based Out-of-Control Management
- Different inspection items can be assigned different SPC decision rules
- Supports 11 out-of-control rules, including the standard Western Electric 8 rules
- Rule parameters are fully configurable
- Rules can be grouped and centrally managed
- Rule groups can be bound to different inspection items, supporting complex manufacturing scenarios
3. Real-Time Dynamic SPC Charts
- SPC charts update automatically in real time
- No browser refresh required when new data arrives
4. Inspection Items & SPC Analysis
Once the quality data is fully and automatically collected, all the data is stored in our SPC system, each inspection item can generate a complete SPC analysis report with a single click, including:
- Appropriate control chart selection and subgroup distribution
- Process Capability Analysis Report
- Cpk, Ppk, PPM
- Distribution charts and fitted curves
- Normal probability plots and capability comparison charts
- Normality tests and distribution fitting tests
- Machine-learning-based anomaly detection charts
- Statistical summary tables and capability tables
- Raw data details and rainbow charts
- Historical out-of-control events and corresponding improvement actions
Supported SPC Analysis Types
Individual data
- I–MR charts
Fixed subgroup
- Xbar charts
- R charts
- S charts
- MR–R/S charts
- Xbar moving range charts
Variable subgroup size
- Xbar charts
- R charts
- S charts
Attribute data
- NP charts
- C charts
- P charts
- U charts
Record-only items
- Descriptive inspection items (for traceability without SPC calculations)
Corrective & Improvement Actions
- Supports documenting corrective actions and resolutions for each SPC violation
- Quick access to historical alarms and their associated corrective actions
Alarm History
- Complete traceability of all historical SPC alarms
- Search and review alarms by inspection item and time period
Inspection Item Comparison
- Compare multiple inspection items side by side
- Identify quality differences across products, machines, lines, or stations
Batch Export of SPC Reports
- Select multiple inspection items at once
- One-click batch export of SPC analysis reports to Excel
- Significantly improves reporting and analysis efficiency
Large Language Model (AI) Support
- Integration with public large language models
- Supports private LLM deployment, such as DeepSeek
- AI-assisted quality analysis, anomaly interpretation, and improvement support
5. SPC Dashboards
As manufacturing digitalization advances, dashboards are widely used on the shop floor to produce production status, such as order progress and workstation load.
Quality management follows the same visualization approach by:
- Deploy real-time SPC dashboards at any production station or line
- Monitor quality anomalies for specific workstations, lines, or orders
Dashboard Capabilities
- Unlimited number of dashboards
- Unlimited charts per dashboard
- Each dashboard has a dedicated URL, ideal for large-screen displays
- Supported visualizations:
- SPC control charts
- Pre-control (rainbow) charts
- Capability histograms
- All charts update in real time
Dashboard Types
- Real-time dashboards
- Comprehensive dashboards
- Statistical dashboards and custom charts
6. SPC Monitoring & Alerts
1)Monitoring Configuration
The anomaly detection item list notification has been upgraded to allow configuration of different notification object groups based on the detection item category.
2)Alert Capabilities
3)Flexible alarm rules
4)Alarm lists and alarm history
5)Email notifications
6)Enterprise WeChat notifications
7. Data Collection Methods
Manual Entry
- SPC control charts are updated instantly in real time upon manual data entry.
Multi-Item Entry
- Enter multiple inspection items simultaneously, even with different data types
Excel Batch Upload
- Batch upload inspection data using a specified template.
Mobile Data Entry
MQTT Data Acquisition
- Collect real-time data from inspection and production equipment via MQTT
HTTP API Integration
- Synchronize data from existing business systems without re-entry
Active JSON Data Collection
SAP Inspection Plan Synchronization
8. Advanced Analysis Tools
We often need to periodically analyze SPC process data for various testing items, or compare process data from different testing items. A fast statistical tool is essential.
SPC Historical Analysis
- Analyze SPC data by configurable time periods
ü Define start and end dates
ü Aggregate by year, quarter, month, week, or day
- Select key metrics such as Cpk and number of rule violations
- Compare multiple inspection items
- Export results or visualize using charts
Box Plot Analysis
- Generate box plots for individual data at fixed time intervals
Correlation Analysis
The SPC system stores a lot of testing data. Some testing items from common sources may be correlated. Generally, we organize this testing data and use Minitab or Excel to perform correlation and regression analyses in pairs, adjusting the lag period to find the optimal antecedent effects. However, a more efficient method is essential:
- Perform scatter plots and regression analysis across multiple inspection items
- Align data by inspection dates
- Define lag ranges to automatically identify preceding effects
- No need to export data to Excel or Minitab—analysis is completed within one system
- Traverse N inspection items × M lag periods to identify correlated test items.
Cpk Tool
- Perform SPC control charting and capability analysis without creating inspection items
- Supports individual and subgroup data
- Paste data directly from Excel
- Replaces traditional control chart and capability analysis functions of Minitab
Regression analysis
ANOVA
- Normality tests
- Distribution fitting
- One-sample t-test
- Two-sample t-test
9. MSA Support
- Gage linearity and bias studies
- Gage R&R (nested)
- Gage R&R (crossed)
- Attribute consistency analysis
10. Application Cases
1) Mettler-Toledo Checkweighers
This is an automatic weighing device. Each weighing sends weight data via a TCP server. We developed a small program:
ü Custom TCP client collects and synchronizes data to SPC system for real-time analysis
2) Bluetooth Micrometers
3) Quality Dimension in Production Scheduling
ü Different sources (machines A or machines B, shifts, lines, operators, etc.) tracked separately
ü Cpk comparison across sources
ü High-priority customers assigned to higher-Cpk production sources (the image below shows machines A and B).
4) Proactive Data Acquisition from DCS Database
ü Large amounts of data have been collected in the data center. Data is directly linked to the database and synchronized to the SPC software.
11. Additional Features
Integration with other business systems via:
ü SSO
ü Token-based URL access
Multi-language Support
ü Supports 11 languages: English, Simplified Chinese, Traditional Chinese, Thai, Japanese, Vietnamese, Korean, Indonesian, Spanish, Hindi, and Malay.
20 theme color options
Others
ü Support:Unlimited users, inspection items, and dashboards
ü Alarm notifications via: Email、API、Enterprise WeChat、DingTalk
Open SPC rule APIs and data APIs
Customizable SPC Analysis Report Styles
ü Customizable SPC report templates
Style groups for different inspection item types
ü Custom alarm notification recipients
- Visitor mode:
- Hides all alarms and abnormal points
- Ideal for audits, customer visits, and demonstrations
User-defined anomaly detection rules
Custom fields and custom data-entry fields
Personalized home page
Simple SPC 4.0 Official Release
More Powerful. More Efficient. Built for Complex Quality Scenarios.

Following its recognition as a Recommended SPC Software in 2025, Bingo SPC is proud to announce the official release of SPC 4.0 in early 2026.
This major upgrade represents a significant leap forward—not only in statistical rigor, but also in operational efficiency, system scalability, and enterprise-grade usability. SPC 4.0 is designed to support more complex manufacturing scenarios while delivering faster insights and stronger process control.
1. Statistical Core: Built for Complex and Real-World Scenarios
Key focus: Breaking the limitations of fixed data structures
- Support for Variable Subgroup Sizes
SPC 4.0 fully supports non-fixed subgroup data. All SPC reports, control chart algorithms, and monitoring modules have been upgraded accordingly, effectively addressing real-world sampling variability in production environments. - Expanded Control Chart Algorithms
New control charts, including MR-R/S Mean Charts and Moving Range Mean Charts, have been introduced to enhance statistical analysis for small samples and variable subgroups. - Text-Based Data Traceability
A new Text Data Type (Type 8) has been added, enabling the recording, upload, and traceability of non-numerical quality information—extending SPC coverage beyond purely quantitative data.
2. Analytical Tools: From Single Calculations to Advanced Diagnostics
Key focus: A productivity multiplier for Quality Engineers
- Enhanced CPK “All-in-One” Analysis
The CPK analysis tool has been fully redesigned into a six-in-one diagnostic report, generating control charts, normal probability plots, capability comparison charts, histograms, and run charts in a single execution. - Simulation and Capability Forecasting
Users can now generate simulated datasets based on target CPK values, supporting process capability estimation and scenario analysis. - Interactive Statistical Analysis
Regression analysis, ANOVA, and t-tests now leverage interactive, spreadsheet-style interfaces, allowing samples to be selected directly from raw data for near-instant analysis. - Optimized Visualization Performance
Box plots, distribution fitting, and related charts now support significantly larger datasets with improved rendering performance and additional reference line options.
3. Monitoring and Alerts: From Detection to Closed-Loop Control
Key focus: Turning signals into actions
- Alert Frequency Optimization
A new minimum alert interval prevents notification overload in high-frequency data environments. Historical alerts retain the corresponding specification limits and capability indices at the time of triggering. - Multi-Dimensional Alert Strategies
Alerts can now be triggered based on SPC out-of-control signals or insufficient CPK/PPK capability, with flexible recipient grouping and retry mechanisms. - Closed-Loop Exception Management
Improvement actions are deeply linked to abnormal events, with support for response rate statistics and logical deletion of resolved abnormal points—strengthening end-to-end quality issue closure.
4. Dashboards & Visualization: Multi-Dimensional Insights
Key focus: Clear visibility for informed decisions
- Statistical Dashboard (New Module)
A dedicated management-level dashboard provides a comprehensive overview of SPC operations across the organization. Highly flexible chart definitions can be created using custom SQL, enabling enterprise-wide monitoring. - Dynamic and Composite Dashboards
Dashboards support automatic and manual switching modes, merged page displays, and configurable page dwell times. Dashboard categories and role-based access control are fully supported.
5. Data Entry & Collection: Seamless Multi-Device Collaboration
Key focus: Real-time accuracy and efficiency
- Multi-Platform Data Entry
A new mobile data entry interface has been introduced. Manual entry screens now provide real-time feedback on process capability indicators (CPK/PPK). - Full Data-Type Coverage
Enhanced support for descriptive inspection items and variable subgroup inspection data. - Advanced Automated Data Collection
New JSON-based data acquisition configurations and direct synchronization with SAP inspection plans are supported. - Measurement Device Integration
Web serial port technology enables direct acquisition of data from electronic gauges and measuring instruments, automatically populating data entry forms.
6. System Foundation: Performance, Stability, and Security
Key focus: Faster, stronger, and more secure
- Performance Enhancements
Bulk Excel data uploads are now up to 10× faster. Database connection pool optimization eliminates interface freezing under high-concurrency conditions. - Global Time Zone Support
User-level time zone configuration ensures accurate timestamp handling across global and multi-site deployments. - Built-in System Maintenance
Integrated log cleanup, monitoring image management, and SFTP-based remote data backup simplify system maintenance. - Granular Access Control
Fine-grained authorization for dashboards and pages by category, with global administrative override support for Admin accounts.
SPC 4.0 Is Ready
SPC 4.0 is now fully available.
We recommend starting with the enhanced CPK six-in-one analysis report and the new Statistical Dashboard to experience the most significant improvements firsthand.
Using data to safeguard safety: Application practices of SPC in the pharmaceutical industry
I. Pharmaceutical Manufacturing: Why Process Stability Matters
In the pharmaceutical industry, the consequences of quality issues extend far beyond economic loss. They directly affect patient safety and expose companies to significant regulatory and compliance risks.
Compared with other manufacturing sectors, pharmaceutical production has several defining characteristics:
Highly complex processes with numerous variables
Extremely stringent quality requirements with minimal allowable variability
Any abnormality may result in batch rejection, production shutdowns, or product recalls
Strict regulatory oversight under frameworks such as GMP and authorities including the NMPA, FDA, and EMA



As a result, the core challenge of pharmaceutical quality management is not simply whether a product meets specifications, but whether:
The manufacturing process remains consistently controlled, stable, and predictable.
This is precisely where Statistical Process Control (SPC) delivers its fundamental value in the pharmaceutical industry.
II. The Role of SPC in Pharmaceutical Manufacturing
In pharmaceutical manufacturing, SPC is far more than a basic statistical quality tool. It serves as:
A critical method for maintaining continuous process control
A key data foundation within GMP systems
A vital bridge connecting processes, equipment, quality, and regulatory compliance
By continuously monitoring Critical Process Parameters (CPPs) and Critical Quality Attributes (CQAs), SPC enables pharmaceutical companies to:
Detect abnormal trends at an early stage
Prevent deviations from escalating into quality incidents
Provide objective, data-driven evidence for deviation investigations and CAPA activities
III. Typical Application Scenarios of SPC in Pharmaceutical Manufacturing
1. Raw Materials and Pre-processing
In active pharmaceutical ingredient (API) and finished dosage manufacturing, SPC is commonly applied to monitor:
Key physicochemical attributes of raw and excipient materials
Particle size distribution and moisture content
Weighing accuracy and variability
SPC allows early identification of abnormal fluctuations in raw materials or pre-processing steps, preventing issues from propagating downstream into subsequent processes.
2. Finished Dosage Manufacturing Process Control
In solid and liquid dosage form manufacturing, SPC is widely used to monitor:
Mixing time and blend uniformity
Tablet weight, hardness, and thickness
Fill volume accuracy and sealing quality
SPC helps distinguish between:
Random variation, and
Systematic shifts or equipment-related abnormalities,
thereby reducing batch-to-batch variability and ensuring consistent product quality.
3. Aseptic and Cleanroom-Related Processes
For sterile products and biopharmaceutical manufacturing, SPC plays a particularly critical role in monitoring:
Environmental conditions (temperature, humidity, microbial levels, particle counts)
Sterilization process parameters
Operating status of critical equipment
Trend-based control charts enable early detection of potential loss-of-control conditions, helping prevent sterility failures before they occur.
4. Packaging and Labeling Processes
During the packaging stage, SPC is commonly applied to:
Fill consistency
Seal integrity
Label positioning and readability
Effective process control at this stage significantly reduces compliance risks related to mislabeling, underfilling, or packaging defects.
IV. Key Characteristics of SPC in the Pharmaceutical Industry
1. Strong Compliance Orientation
SPC data is frequently used to support:
GMP audits and inspections
Deviation investigations
Verification of CAPA effectiveness
By translating abstract GMP requirements into measurable and continuously monitored process indicators, SPC plays a critical role across all quality assurance activities. Data integrity, traceability, and audit readiness are especially essential.
2. Greater Focus on Trends Rather Than Single Limit Exceedances
In pharmaceutical manufacturing, many quality risks do not arise from isolated out-of-specification events, but from:
Gradual and long-term process drift.
SPC trend analysis enables proactive intervention before deviations formally occur.
3. Integration with Validation and Continued Process Verification
SPC is often implemented in conjunction with:
Process Validation (PV)
Continued Process Verification (CPV)
forming a core component of lifecycle process management.
V. Core Value Delivered by SPC to Pharmaceutical Companies
Improved process stability and product consistency
Reduced risk of batch deviations and product rejection
Stronger support for GMP compliance and regulatory audits
More efficient deviation handling and CAPA execution
Establishment of a data-driven quality culture
As regulatory expectations continue to increase, SPC has evolved from an optional tool into a foundational capability within pharmaceutical quality systems.
VI. Conclusion: SPC as the “Second Line of Defense” in Pharmaceutical Quality
In the pharmaceutical industry:
Compliance is the baseline. Stability is the core. Data is the safeguard.
Through continuous monitoring and trend analysis, SPC enables manufacturers to take action before problems occur—protecting patient safety, reducing operational risk, and supporting long-term, stable production.
Truly mature pharmaceutical manufacturing does not rely on end-product testing alone, but on controlled and predictable processes.
- What If Subgroup Sizes Are Not Fixed?
- Invisible Variations That Define Yield :Practical Applications of SPC in Semiconductor Manufacturing
- Simple SPC 4.0 – Detailed Product Overview
- Simple SPC 4.0 Official Release
- Using data to safeguard safety: Application practices of SPC in the pharmaceutical industry
- How can risks be identified in advance in automobile manufacturing? — Sharing SPC application practices
- Innovative Practices and Applications of Web SPC Systems
- Should Manufacturing Companies Still Implement SPC Amid Economic Downturn and Poor Business Performance?
- What should we do if the customer requires the immediate implementation of SPC process control during a factory audit?
- Simple SPC has been recognized for the third time as the "2025 SPC Statistical Process Control Software of the Year" by China SoftWare Home.
- Giving SPC AI Wings: DeepSeek Enhancing Efficiency and Depth of Quality Management
- AI-Enhanced Statistical Process Control (AI-SPC): Revolutionizing Quality Management in the Era of Smart Manufacturing
- General-Purpose Artificial Intelligence Models (DeepSeek, etc.) and Statistical Process Control (SPC): A New Era of Intelligent Quality Management
- Simple SPC 2.0 released, with upgraded functions and optimized performance
- CPK and PPK: Essential Questions in Quality Interviews, Do You Truly Understand Them?
- Unilateral or Bilateral: An In-Depth Exploration of Specification Limits and Control Limits in SPC Analysis and Their Impact on Metrics
- How to Calculate Control Limits for Xbar-R and Xbar-S Control Charts in SPC Analysis and When to Use Each Chart
- Is SPC or Another Method Better for Determining Batch Consistency with Standards? A Recommended Analysis Approach
- Beyond SPC Control Charts: Lesser-Known but Effective Quality Analysis Tools
- SPC is the most accessible, effective, and performance-demonstrating analytical tool in the manufacturing industry.
- How to Quickly Identify Hidden Correlations Between Test Items Using the SPC System?